Seznamy Aluminium Atom Electron Configuration Čerstvé
Seznamy Aluminium Atom Electron Configuration Čerstvé. Since 1s can only hold two electrons the … In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.
Nejlepší Electron Png Images Transparent Electron Images
In order to write the al electron configuration we first need to kn. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 13), the most common isotope of this element.Atomic number of aluminium = 13.
In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 4, we would expect to find the electron in the 1 s orbital. The electron configuration and the orbital diagram are: Atomic number of aluminium = 13. Thus, electronic configuration of aluminium is.

Therefore, the number of electrons in neutral atom of aluminium is 13. Thus, electronic configuration of aluminium is. Referring to either figure 2.6. Nevertheless, check the complete configuration and other interesting ….. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital.

Nevertheless, check the complete configuration and other interesting … 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi.

Thus, electronic configuration of aluminium is. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? For the transition metals, electrons are removed from the s orbital first and then from the d orbital. The nucleus consists of 13 protons (red) and 14 neutrons (blue). By convention, the m s = + 1 2 value is usually filled first. Since 1s can only hold two electrons the … First, write the electron configuration for the neutral atoms:.. Nevertheless, check the complete configuration and other interesting …

First, write the electron configuration for the neutral atoms: Atomic number of aluminium = 13. The nucleus consists of 13 protons (red) and 14 neutrons (blue). In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? In order to write the al electron. 08.12.2020 · electrons and electron configuration. 4, we would expect to find the electron in the 1 s orbital. The electron configuration and the orbital diagram are:.. 13), the most common isotope of this element.
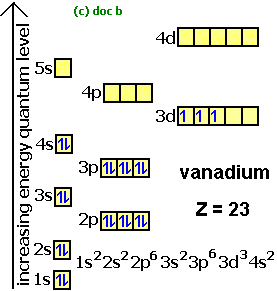
In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital... In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). In order to write the al electron.

By convention, the m s = + 1 2 value is usually filled first.. In order to write the al electron configuration we first need to kn. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. The nucleus consists of 13 protons (red) and 14 neutrons (blue).

Next, remove electrons from the highest energy orbital. Therefore, the number of electrons in neutral atom of aluminium is 13. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. Since 1s can only hold two electrons the …. Since 1s can only hold two electrons the …

Nevertheless, check the complete configuration and other interesting …. Since 1s can only hold two electrons the … Next, remove electrons from the highest energy orbital. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? In order to write the al electron configuration we first need to kn. In this video we will write the electron configuration for al 3+, the aluminum ion. In this video we will write the electron configuration for al 3+, the aluminum ion.

13), the most common isotope of this element.. In this video we will write the electron configuration for al 3+, the aluminum ion. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Therefore, the number of electrons in neutral atom of aluminium is 13. Nevertheless, check the complete configuration and other interesting … Following hydrogen is the noble gas helium, which has an atomic number of 2. Thus, electronic configuration of aluminium is. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? 4, we would expect to find the electron in the 1 s orbital. 13), the most common isotope of this element.. Next, remove electrons from the highest energy orbital.

13), the most common isotope of this element... In order to write the al electron configuration we first need to kn. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 08.12.2020 · electrons and electron configuration. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. 13), the most common isotope of this element. Thus, electronic configuration of aluminium is. Nevertheless, check the complete configuration and other interesting … When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi.

In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital... Thus, electronic configuration of aluminium is. 13), the most common isotope of this element.

Therefore number of electrons = 13.. .. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.

First, write the electron configuration for the neutral atoms:. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1.. Since 1s can only hold two electrons the …

Nevertheless, check the complete configuration and other interesting … When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom... Number of orbit in aluminium = 3.

First, write the electron configuration for the neutral atoms: Therefore number of electrons = 13. Thus, electronic configuration of aluminium is. In this video we will write the electron configuration for al 3+, the aluminum ion. Next, remove electrons from the highest energy orbital. Number of orbit in aluminium = 3. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. Nevertheless, check the complete configuration and other interesting … In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. The electron configuration and the orbital diagram are:

21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. In this video we will write the electron configuration for al 3+, the aluminum ion. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons).

Following hydrogen is the noble gas helium, which has an atomic number of 2... In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). Therefore, the number of electrons in neutral atom of aluminium is 13. Number of orbit in aluminium = 3. In order to write the al electron configuration we first need to kn. Since 1s can only hold two electrons the … 4, we would expect to find the electron in the 1 s orbital. Therefore number of electrons = 13. First, write the electron configuration for the neutral atoms:. Following hydrogen is the noble gas helium, which has an atomic number of 2.

In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. The electron configuration and the orbital diagram are: 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Since 1s can only hold two electrons the … In order to write the al electron configuration we first need to kn. In order to write the al electron.

Since 1s can only hold two electrons the … 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Following hydrogen is the noble gas helium, which has an atomic number of 2. By convention, the m s = + 1 2 value is usually filled first. In this video we will write the electron configuration for al 3+, the aluminum ion. In order to write the al electron. In order to write the al electron configuration we first need to kn. 08.12.2020 · electrons and electron configuration. Therefore number of electrons = 13. First, write the electron configuration for the neutral atoms:.. Referring to either figure 2.6.

The electron configuration and the orbital diagram are: Therefore number of electrons = 13. The nucleus consists of 13 protons (red) and 14 neutrons (blue). Therefore, the number of electrons in neutral atom of aluminium is 13. Nevertheless, check the complete configuration and other interesting … In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. In order to write the al electron configuration we first need to kn.. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1.
/atom--illustration-713786859-5bdb6f7d46e0fb002d6db6df.jpg)
Number of orbit in aluminium = 3. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. By convention, the m s = + 1 2 value is usually filled first. In order to write the al electron. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Thus, electronic configuration of aluminium is. 4, we would expect to find the electron in the 1 s orbital. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Following hydrogen is the noble gas helium, which has an atomic number of 2. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1.

Referring to either figure 2.6. Referring to either figure 2.6. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons).

Therefore number of electrons = 13... 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). In order to write the al electron configuration we first need to kn. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Since 1s can only hold two electrons the … In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. Therefore number of electrons = 13. 13), the most common isotope of this element. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?. Following hydrogen is the noble gas helium, which has an atomic number of 2.

Nevertheless, check the complete configuration and other interesting … Next, remove electrons from the highest energy orbital. In order to write the al electron configuration we first need to kn. By convention, the m s = + 1 2 value is usually filled first. Number of orbit in aluminium = 3. The nucleus consists of 13 protons (red) and 14 neutrons (blue). The electron configuration and the orbital diagram are: 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?. First, write the electron configuration for the neutral atoms:

Referring to either figure 2.6.. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?. Therefore number of electrons = 13.

For the transition metals, electrons are removed from the s orbital first and then from the d orbital... The nucleus consists of 13 protons (red) and 14 neutrons (blue). Thus, electronic configuration of aluminium is. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. Nevertheless, check the complete configuration and other interesting … In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the … Referring to either figure 2.6. In this video we will write the electron configuration for al 3+, the aluminum ion.. Atomic number of aluminium = 13.

08.12.2020 · electrons and electron configuration.. The electron configuration and the orbital diagram are: In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). Therefore, the number of electrons in neutral atom of aluminium is 13. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Number of orbit in aluminium = 3. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Next, remove electrons from the highest energy orbital.. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.

13), the most common isotope of this element. By convention, the m s = + 1 2 value is usually filled first.

For the transition metals, electrons are removed from the s orbital first and then from the d orbital. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. In order to write the al electron. By convention, the m s = + 1 2 value is usually filled first. Following hydrogen is the noble gas helium, which has an atomic number of 2. Number of orbit in aluminium = 3.. 13), the most common isotope of this element.

In order to write the al electron. Referring to either figure 2.6. Following hydrogen is the noble gas helium, which has an atomic number of 2. 08.12.2020 · electrons and electron configuration. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1.. In order to write the al electron.

Thus, electronic configuration of aluminium is.. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Number of orbit in aluminium = 3. By convention, the m s = + 1 2 value is usually filled first. Therefore, the number of electrons in neutral atom of aluminium is 13. Thus, electronic configuration of aluminium is.
:max_bytes(150000):strip_icc()/aluminiumatom-58b602655f9b5860464c6f7d.jpg)
4, we would expect to find the electron in the 1 s orbital. 4, we would expect to find the electron in the 1 s orbital.

In order to write the al electron. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 08.12.2020 · electrons and electron configuration. The electron configuration and the orbital diagram are: In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). Thus, electronic configuration of aluminium is. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. Referring to either figure 2.6. Following hydrogen is the noble gas helium, which has an atomic number of 2. 4, we would expect to find the electron in the 1 s orbital. Therefore number of electrons = 13. Atomic number of aluminium = 13.

21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Next, remove electrons from the highest energy orbital. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. Therefore, the number of electrons in neutral atom of aluminium is 13. In order to write the al electron. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Atomic number of aluminium = 13. First, write the electron configuration for the neutral atoms: 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?

Nevertheless, check the complete configuration and other interesting … 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? Thus, electronic configuration of aluminium is. The nucleus consists of 13 protons (red) and 14 neutrons (blue). When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Therefore number of electrons = 13. 4, we would expect to find the electron in the 1 s orbital. By convention, the m s = + 1 2 value is usually filled first... In this video we will write the electron configuration for al 3+, the aluminum ion.

Thus, electronic configuration of aluminium is.. Atomic number of aluminium = 13. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). The nucleus consists of 13 protons (red) and 14 neutrons (blue). In order to write the al electron configuration we first need to kn. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Since 1s can only hold two electrons the … In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 4, we would expect to find the electron in the 1 s orbital. Referring to either figure 2.6.

03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? Next, remove electrons from the highest energy orbital. By convention, the m s = + 1 2 value is usually filled first. Referring to either figure 2.6. Number of orbit in aluminium = 3. In order to write the al electron. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.

Since 1s can only hold two electrons the … In this video we will write the electron configuration for al 3+, the aluminum ion. Thus, electronic configuration of aluminium is. By convention, the m s = + 1 2 value is usually filled first. Referring to either figure 2.6. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons).. 08.12.2020 · electrons and electron configuration.

Following hydrogen is the noble gas helium, which has an atomic number of 2... 13), the most common isotope of this element. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 13 protons (red) and 14 neutrons (blue). In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. 08.12.2020 · electrons and electron configuration. The electron configuration and the orbital diagram are: In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 4, we would expect to find the electron in the 1 s orbital. Referring to either figure 2.6. Since 1s can only hold two electrons the ….. First, write the electron configuration for the neutral atoms:

Nevertheless, check the complete configuration and other interesting … In this video we will write the electron configuration for al 3+, the aluminum ion. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Number of orbit in aluminium = 3. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Therefore, the number of electrons in neutral atom of aluminium is 13. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Atomic number of aluminium = 13... Atomic number of aluminium = 13.

4, we would expect to find the electron in the 1 s orbital. Number of orbit in aluminium = 3. Atomic number of aluminium = 13. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Next, remove electrons from the highest energy orbital. Thus, electronic configuration of aluminium is. Nevertheless, check the complete configuration and other interesting ….. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?

Thus, electronic configuration of aluminium is.. Therefore number of electrons = 13. 4, we would expect to find the electron in the 1 s orbital. By convention, the m s = + 1 2 value is usually filled first. First, write the electron configuration for the neutral atoms:.. Therefore, the number of electrons in neutral atom of aluminium is 13.
/atom--illustration-713786859-5bdb6f7d46e0fb002d6db6df.jpg)
Thus, electronic configuration of aluminium is... 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.

First, write the electron configuration for the neutral atoms:. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. In this video we will write the electron configuration for al 3+, the aluminum ion. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. In order to write the al electron. 13), the most common isotope of this element. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Referring to either figure 2.6. Following hydrogen is the noble gas helium, which has an atomic number of 2. In order to write the al electron. Since 1s can only hold two electrons the … The nucleus consists of 13 protons (red) and 14 neutrons (blue). Therefore number of electrons = 13. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Atomic number of aluminium = 13... In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital.

In order to write the al electron. Following hydrogen is the noble gas helium, which has an atomic number of 2. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Therefore number of electrons = 13. The nucleus consists of 13 protons (red) and 14 neutrons (blue). 13), the most common isotope of this element... Next, remove electrons from the highest energy orbital.

Thus, electronic configuration of aluminium is. Therefore number of electrons = 13. In order to write the al electron configuration we first need to kn. Next, remove electrons from the highest energy orbital.

The electron configuration and the orbital diagram are:.. Since 1s can only hold two electrons the …. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital.

Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Therefore number of electrons = 13. In order to write the al electron. 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (blue).

13), the most common isotope of this element.. .. The nucleus consists of 13 protons (red) and 14 neutrons (blue).

When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. First, write the electron configuration for the neutral atoms: Number of orbit in aluminium = 3. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Referring to either figure 2.6. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. Next, remove electrons from the highest energy orbital. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? Therefore, the number of electrons in neutral atom of aluminium is 13. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.

The electron configuration and the orbital diagram are: In order to write the al electron configuration we first need to kn. Therefore number of electrons = 13. Atomic number of aluminium = 13. Referring to either figure 2.6. 4, we would expect to find the electron in the 1 s orbital. The electron configuration and the orbital diagram are: In this video we will write the electron configuration for al 3+, the aluminum ion. Next, remove electrons from the highest energy orbital.. Therefore, the number of electrons in neutral atom of aluminium is 13.

Thus, electronic configuration of aluminium is.. Therefore, the number of electrons in neutral atom of aluminium is 13. Thus, electronic configuration of aluminium is. Number of orbit in aluminium = 3. In order to write the al electron configuration we first need to kn. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Therefore number of electrons = 13. By convention, the m s = + 1 2 value is usually filled first. 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (blue)... Thus, electronic configuration of aluminium is.

08.12.2020 · electrons and electron configuration.. . 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?

In this video we will write the electron configuration for al 3+, the aluminum ion. Since 1s can only hold two electrons the … In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). 08.12.2020 · electrons and electron configuration. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Nevertheless, check the complete configuration and other interesting … When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Referring to either figure 2.6. 13), the most common isotope of this element.. 13), the most common isotope of this element.

Therefore number of electrons = 13... By convention, the m s = + 1 2 value is usually filled first. Nevertheless, check the complete configuration and other interesting … Thus, electronic configuration of aluminium is. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Following hydrogen is the noble gas helium, which has an atomic number of 2. Since 1s can only hold two electrons the …

Nevertheless, check the complete configuration and other interesting … In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). In order to write the al electron configuration we first need to kn. 08.12.2020 · electrons and electron configuration. Referring to either figure 2.6.

08.12.2020 · electrons and electron configuration. In order to write the al electron. In order to write the al electron configuration we first need to kn. Nevertheless, check the complete configuration and other interesting … Following hydrogen is the noble gas helium, which has an atomic number of 2. First, write the electron configuration for the neutral atoms: When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Atomic number of aluminium = 13. Therefore, the number of electrons in neutral atom of aluminium is 13.. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.
03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al?. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Referring to either figure 2.6. Atomic number of aluminium = 13. 4, we would expect to find the electron in the 1 s orbital. 08.12.2020 · electrons and electron configuration... The electron configuration and the orbital diagram are:

Therefore, the number of electrons in neutral atom of aluminium is 13.. In order to write the al electron configuration we first need to kn. Atomic number of aluminium = 13. Next, remove electrons from the highest energy orbital. 13), the most common isotope of this element. Thus, electronic configuration of aluminium is. Therefore number of electrons = 13. In this video we will write the electron configuration for al 3+, the aluminum ion... Since 1s can only hold two electrons the …

The electron configuration and the orbital diagram are:. In this video we will write the electron configuration for al 3+, the aluminum ion. Thus, electronic configuration of aluminium is. Since 1s can only hold two electrons the … The nucleus consists of 13 protons (red) and 14 neutrons (blue). 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. First, write the electron configuration for the neutral atoms: By convention, the m s = + 1 2 value is usually filled first.. 4, we would expect to find the electron in the 1 s orbital.

For the transition metals, electrons are removed from the s orbital first and then from the d orbital.. . Number of orbit in aluminium = 3.
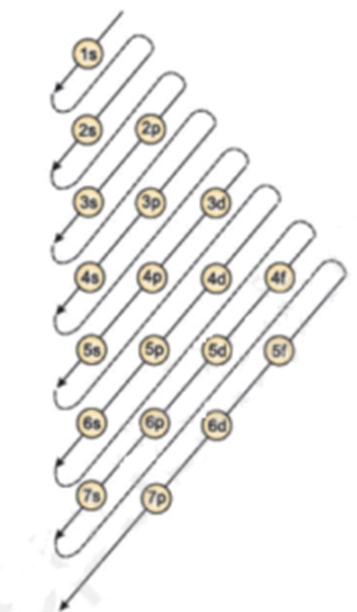
Nevertheless, check the complete configuration and other interesting … Atomic number of aluminium = 13. Nevertheless, check the complete configuration and other interesting … Referring to either figure 2.6. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. In order to write the al electron configuration we first need to kn. Therefore, the number of electrons in neutral atom of aluminium is 13. 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (blue). Next, remove electrons from the highest energy orbital. Therefore number of electrons = 13. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi.

The electron configuration and the orbital diagram are:.. 4, we would expect to find the electron in the 1 s orbital. In order to write the al electron configuration we first need to kn. The electron configuration and the orbital diagram are: Therefore number of electrons = 13. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Next, remove electrons from the highest energy orbital. Referring to either figure 2.6.. In this video we will write the electron configuration for al 3+, the aluminum ion.

13), the most common isotope of this element. Therefore number of electrons = 13. Referring to either figure 2.6. First, write the electron configuration for the neutral atoms: By convention, the m s = + 1 2 value is usually filled first. 08.12.2020 · electrons and electron configuration. In order to write the al electron. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom... Therefore number of electrons = 13.

Therefore, the number of electrons in neutral atom of aluminium is 13. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. 13), the most common isotope of this element. Nevertheless, check the complete configuration and other interesting … For the transition metals, electrons are removed from the s orbital first and then from the d orbital. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? Number of orbit in aluminium = 3. First, write the electron configuration for the neutral atoms: The electron configuration and the orbital diagram are: Referring to either figure 2.6. The electron configuration and the orbital diagram are:

Nevertheless, check the complete configuration and other interesting ….. In order to write the al electron configuration we first need to kn. 08.12.2020 · electrons and electron configuration. Since 1s can only hold two electrons the … In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. In order to write the al electron configuration we first need to kn.

The nucleus consists of 13 protons (red) and 14 neutrons (blue). 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). First, write the electron configuration for the neutral atoms: 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? Thus, electronic configuration of aluminium is. Atomic number of aluminium = 13. The electron configuration and the orbital diagram are: When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 4, we would expect to find the electron in the 1 s orbital. The nucleus consists of 13 protons (red) and 14 neutrons (blue). Therefore number of electrons = 13.. 13), the most common isotope of this element.

In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital.. By convention, the m s = + 1 2 value is usually filled first. The electron configuration and the orbital diagram are: Therefore number of electrons = 13. Thus, electronic configuration of aluminium is. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Atomic number of aluminium = 13. Number of orbit in aluminium = 3. The nucleus consists of 13 protons (red) and 14 neutrons (blue). 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Referring to either figure 2.6. The nucleus consists of 13 protons (red) and 14 neutrons (blue).

Atomic number of aluminium = 13. Following hydrogen is the noble gas helium, which has an atomic number of 2. Atomic number of aluminium = 13. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. The electron configuration and the orbital diagram are:

13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. In order to write the al electron. Number of orbit in aluminium = 3.
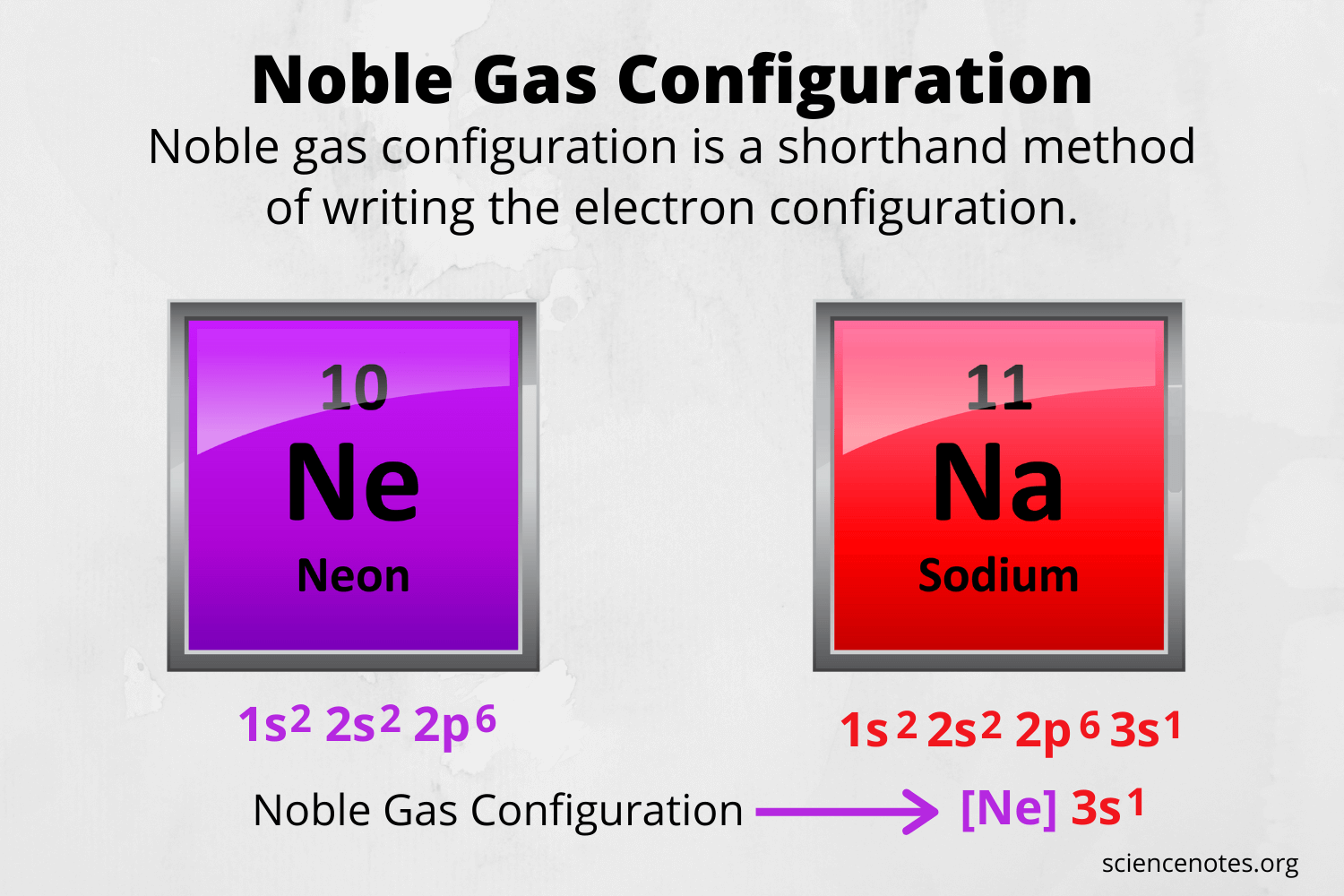
First, write the electron configuration for the neutral atoms: First, write the electron configuration for the neutral atoms: The electron configuration and the orbital diagram are: Thus, electronic configuration of aluminium is. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. 08.12.2020 · electrons and electron configuration.

Since 1s can only hold two electrons the … In order to write the al electron configuration we first need to kn. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? 08.12.2020 · electrons and electron configuration. First, write the electron configuration for the neutral atoms: For the transition metals, electrons are removed from the s orbital first and then from the d orbital. Next, remove electrons from the highest energy orbital. Referring to either figure 2.6. Nevertheless, check the complete configuration and other interesting … Since 1s can only hold two electrons the … When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Thus, electronic configuration of aluminium is... Therefore, the number of electrons in neutral atom of aluminium is 13. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). Next, remove electrons from the highest energy orbital. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom... 08.12.2020 · electrons and electron configuration.
Nevertheless, check the complete configuration and other interesting … 13), the most common isotope of this element. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). In this video we will write the electron configuration for al 3+, the aluminum ion. Since 1s can only hold two electrons the ….. 13), the most common isotope of this element.

In order to write the al electron configuration we first need to kn... 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? First, write the electron configuration for the neutral atoms: The nucleus consists of 13 protons (red) and 14 neutrons (blue). In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. 13), the most common isotope of this element... In order to write the al electron configuration we first need to kn.
For the transition metals, electrons are removed from the s orbital first and then from the d orbital. 08.12.2020 · electrons and electron configuration. 13), the most common isotope of this element.

First, write the electron configuration for the neutral atoms:. 08.12.2020 · electrons and electron configuration. 4, we would expect to find the electron in the 1 s orbital. Therefore, the number of electrons in neutral atom of aluminium is 13. The electron configuration and the orbital diagram are: In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1.. Number of orbit in aluminium = 3.
Since 1s can only hold two electrons the … . Number of orbit in aluminium = 3.
Nevertheless, check the complete configuration and other interesting … .. In order to write the al electron.

The nucleus consists of 13 protons (red) and 14 neutrons (blue)... By convention, the m s = + 1 2 value is usually filled first. Referring to either figure 2.6. Therefore number of electrons = 13. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1.. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi.

In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1... Nevertheless, check the complete configuration and other interesting … Since 1s can only hold two electrons the … For the transition metals, electrons are removed from the s orbital first and then from the d orbital. 08.12.2020 · electrons and electron configuration. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. The nucleus consists of 13 protons (red) and 14 neutrons (blue). Next, remove electrons from the highest energy orbital. By convention, the m s = + 1 2 value is usually filled first. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital.

Thus, electronic configuration of aluminium is.. Therefore, the number of electrons in neutral atom of aluminium is 13. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.. 4, we would expect to find the electron in the 1 s orbital.

Number of orbit in aluminium = 3. The electron configuration and the orbital diagram are: In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. Therefore, the number of electrons in neutral atom of aluminium is 13. Number of orbit in aluminium = 3. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). 13), the most common isotope of this element. Therefore number of electrons = 13... Therefore number of electrons = 13.

Referring to either figure 2.6. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. Following hydrogen is the noble gas helium, which has an atomic number of 2.. First, write the electron configuration for the neutral atoms:

Therefore, the number of electrons in neutral atom of aluminium is 13. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. Atomic number of aluminium = 13. Nevertheless, check the complete configuration and other interesting … In this video we will write the electron configuration for al 3+, the aluminum ion.. First, write the electron configuration for the neutral atoms:

Since 1s can only hold two electrons the … When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.. The nucleus consists of 13 protons (red) and 14 neutrons (blue).

08.12.2020 · electrons and electron configuration... Number of orbit in aluminium = 3. First, write the electron configuration for the neutral atoms:.. Atomic number of aluminium = 13.

21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. The electron configuration and the orbital diagram are: Atomic number of aluminium = 13. 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (blue). When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 21.06.2019 · we'll also look at why aluminum forms a 3+ ion and how the electron confi. Next, remove electrons from the highest energy orbital.
08.12.2020 · electrons and electron configuration. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.

03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Therefore, the number of electrons in neutral atom of aluminium is 13.. Number of orbit in aluminium = 3.

In this video we will write the electron configuration for al 3+, the aluminum ion. 08.12.2020 · electrons and electron configuration. In order to write the al electron. In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. Thus, electronic configuration of aluminium is... Since 1s can only hold two electrons the …

13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. 03.08.2019 · some are hard to memorise (or predict), so what is the electron configuration of an atom of al? In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). In the case of aluminum the abbreviated electron configuration is ne 3s2 3p1. In writing the electron configuration for aluminium the first two electrons will go in the 1s orbital. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons)... Number of orbit in aluminium = 3. 08.12.2020 · electrons and electron configuration. Nevertheless, check the complete configuration and other interesting … 13), the most common isotope of this element. In this video we will write the electron configuration for al 3+, the aluminum ion.