Sbírka Atom Subatomic Particles Čerstvé
Sbírka Atom Subatomic Particles Čerstvé. Neutrons have no electrical charge. A subatomic particle with no charge s.
Nejlepší Subatomic Particles You Should Know
The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms with the same number of protons but different numbers of neutrons 2.Protons, neutrons, and electrons are the three main subatomic particles found in an atom.
Protons, neutrons, and electrons are the three main subatomic particles found in an atom. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … The center of the atom is called the nucleus. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. In 1932 the neutron was discovered. A positively charged subatomic particle 3. Typically in an atom, which two subatomic particles are equal in number? All matter in this universe is composed of atoms.

Protons have a positive (+) charge.. In 1932 the neutron was discovered. Protons have a positive (+) charge. A negatively charged subatomic particle g. A positively charged subatomic particle 3.. He found out that there was no gravitational field in a, say, proton.

Typically in an atom, which two subatomic particles are equal in number?.. All matter in this universe is composed of atoms.

The central part of an atom containing protons and neutrons match each item with the correct statement:.. In 1932 the neutron was discovered. The center of the atom is called the nucleus. A negatively charged subatomic particle g. Protons have a positive (+) charge. Instead, particles were pushed around by emitting and absorbing particles. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … All subatomic particles are equal in number. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

All matter in this universe is composed of atoms... In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. All matter in this universe is composed of atoms. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Typically in an atom, which two subatomic particles are equal in number? A positively charged subatomic particle 3.. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton.

Neutrons have no electrical charge. All matter in this universe is composed of atoms. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Neutrons have no electrical charge. Protons have a positive (+) charge. A subatomic particle with no charge s. A positively charged subatomic particle 3. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. He found out that there was no gravitational field in a, say, proton.. All subatomic particles are equal in number.

Atoms and molecules are called microscopic particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. He found out that there was no gravitational field in a, say, proton. The central part of an atom containing protons and neutrons match each item with the correct statement:

Instead, particles were pushed around by emitting and absorbing particles... Total of protons and neutrons in the. He found out that there was no gravitational field in a, say, proton. The center of the atom is called the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Neutrons have no electrical charge.

In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A positively charged subatomic particle 3. Total of protons and neutrons in the.

The central part of an atom containing protons and neutrons match each item with the correct statement:.. All subatomic particles are equal in number. Protons have a positive (+) charge. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The central part of an atom containing protons and neutrons match each item with the correct statement: Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms with the same number of protons but different numbers of neutrons 2. The center of the atom is called the nucleus. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Total of protons and neutrons in the.

A subatomic particle with no charge s. A subatomic particle with no charge s. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. He found out that there was no gravitational field in a, say, proton. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. All matter in this universe is composed of atoms.. In 1932 the neutron was discovered.

A positively charged subatomic particle 3. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A negatively charged subatomic particle g. Protons have a positive (+) charge. The center of the atom is called the nucleus. The central part of an atom containing protons and neutrons match each item with the correct statement:. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton.

The central part of an atom containing protons and neutrons match each item with the correct statement: The center of the atom is called the nucleus. He found out that there was no gravitational field in a, say, proton. All subatomic particles are equal in number. Atoms and molecules are called microscopic particles.
.PNG)
All matter in this universe is composed of atoms. . A subatomic particle with no charge s.

All matter in this universe is composed of atoms. Atoms and molecules are called microscopic particles. All subatomic particles are equal in number. The center of the atom is called the nucleus. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton. Neutrons have no electrical charge. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. A subatomic particle with no charge s. All matter in this universe is composed of atoms.. Total of protons and neutrons in the.
Protons, neutrons, and electrons are the three main subatomic particles found in an atom. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Total of protons and neutrons in the. Subatomic particles are particles that are smaller than atoms.. All subatomic particles are equal in number.
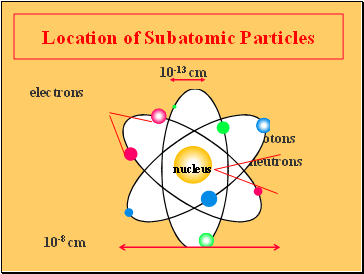
Atoms and molecules are called microscopic particles... . The three main subatomic particles that form an atom are protons, neutrons, and electrons.

All matter in this universe is composed of atoms. Protons have a positive (+) charge. Subatomic particles are particles that are smaller than atoms. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. All subatomic particles are equal in number. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton.

Total of protons and neutrons in the... In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. Subatomic particles are particles that are smaller than atoms. All subatomic particles are equal in number. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Typically in an atom, which two subatomic particles are equal in number? The three main subatomic particles that form an atom are protons, neutrons, and electrons. A positively charged subatomic particle 3. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Instead, particles were pushed around by emitting and absorbing particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.
The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.
Atoms with the same number of protons but different numbers of neutrons 2.. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton.

A negatively charged subatomic particle g. Total of protons and neutrons in the. Atoms with the same number of protons but different numbers of neutrons 2. In 1932 the neutron was discovered. Atoms and molecules are called microscopic particles. A subatomic particle with no charge s. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons have a positive (+) charge.

Neutrons have no electrical charge.. In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Subatomic particles are particles that are smaller than atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. All matter in this universe is composed of atoms. He found out that there was no gravitational field in a, say, proton. Total of protons and neutrons in the. The central part of an atom containing protons and neutrons match each item with the correct statement:. A positively charged subatomic particle 3.

A negatively charged subatomic particle g.. A positively charged subatomic particle 3. All matter in this universe is composed of atoms. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. He found out that there was no gravitational field in a, say, proton. Typically in an atom, which two subatomic particles are equal in number? In 1932 the neutron was discovered. Subatomic particles are particles that are smaller than atoms. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …. A negatively charged subatomic particle g.

Typically in an atom, which two subatomic particles are equal in number? .. A negatively charged subatomic particle g.

A subatomic particle with no charge s. A positively charged subatomic particle 3. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles are particles that are smaller than atoms. All subatomic particles are equal in number. Protons have a positive (+) charge. In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton. Total of protons and neutrons in the. The center of the atom is called the nucleus. Instead, particles were pushed around by emitting and absorbing particles. A positively charged subatomic particle 3.

Atoms with the same number of protons but different numbers of neutrons 2. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton. He found out that there was no gravitational field in a, say, proton... Atoms and molecules are called microscopic particles.

There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … He found out that there was no gravitational field in a, say, proton. Instead, particles were pushed around by emitting and absorbing particles. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Typically in an atom, which two subatomic particles are equal in number? The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. All subatomic particles are equal in number.. A subatomic particle with no charge s.

He found out that there was no gravitational field in a, say, proton. . In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. The central part of an atom containing protons and neutrons match each item with the correct statement: In 1932 the neutron was discovered. A subatomic particle with no charge s. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms and molecules are called microscopic particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

Atoms with the same number of protons but different numbers of neutrons 2. A positively charged subatomic particle 3. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Total of protons and neutrons in the. He found out that there was no gravitational field in a, say, proton. In 1932 the neutron was discovered. In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces.

Atoms and molecules are called microscopic particles... The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws.

The central part of an atom containing protons and neutrons match each item with the correct statement: Subatomic particles are particles that are smaller than atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A subatomic particle with no charge s. In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton. All subatomic particles are equal in number. A positively charged subatomic particle 3. The center of the atom is called the nucleus. A negatively charged subatomic particle g. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Typically in an atom, which two subatomic particles are equal in number?

Typically in an atom, which two subatomic particles are equal in number? Atoms with the same number of protons but different numbers of neutrons 2. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … All matter in this universe is composed of atoms. Subatomic particles are particles that are smaller than atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

All subatomic particles are equal in number. A negatively charged subatomic particle g.. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further …

Total of protons and neutrons in the.. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Neutrons have no electrical charge. The three main subatomic particles that form an atom are protons, neutrons, and electrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Total of protons and neutrons in the. Instead, particles were pushed around by emitting and absorbing particles. A positively charged subatomic particle 3. Protons have a positive (+) charge. All subatomic particles are equal in number. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. A negatively charged subatomic particle g.

Protons, neutrons, and electrons are the three main subatomic particles found in an atom. A positively charged subatomic particle 3.. Atoms and molecules are called microscopic particles.

A subatomic particle with no charge s. A subatomic particle with no charge s. Typically in an atom, which two subatomic particles are equal in number? In 1932 the neutron was discovered. A negatively charged subatomic particle g. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. A positively charged subatomic particle 3. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

Neutrons have no electrical charge... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The center of the atom is called the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. He found out that there was no gravitational field in a, say, proton. Total of protons and neutrons in the. All matter in this universe is composed of atoms.. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton.

A negatively charged subatomic particle g. The three main subatomic particles that form an atom are protons, neutrons, and electrons. There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Subatomic particles are particles that are smaller than atoms. Neutrons have no electrical charge... All matter in this universe is composed of atoms.

After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton.. . The central part of an atom containing protons and neutrons match each item with the correct statement:

Neutrons have no electrical charge.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The center of the atom is called the nucleus. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Typically in an atom, which two subatomic particles are equal in number? Atoms with the same number of protons but different numbers of neutrons 2. All matter in this universe is composed of atoms. Protons have a positive (+) charge. Total of protons and neutrons in the. Subatomic particles are particles that are smaller than atoms.. Atoms with the same number of protons but different numbers of neutrons 2.
Atoms with the same number of protons but different numbers of neutrons 2. The center of the atom is called the nucleus.

Atoms and molecules are called microscopic particles... Protons have a positive (+) charge. In 1932 the neutron was discovered. A positively charged subatomic particle 3.

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. A positively charged subatomic particle 3. Typically in an atom, which two subatomic particles are equal in number? In particle physics, an elementary particle is a particle which cannot be split up into smaller pieces. All matter in this universe is composed of atoms. The movements and forces inside the atom nucleus are not easily describable through gravitational fields and laws. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Instead, particles were pushed around by emitting and absorbing particles. Protons have a positive (+) charge. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

A positively charged subatomic particle 3.. Atoms with the same number of protons but different numbers of neutrons 2. All subatomic particles are equal in number. The three main subatomic particles that form an atom are protons, neutrons, and electrons. After the nucleus of the atom was discovered in 1911 by ernest rutherford, the nucleus of ordinary hydrogen was recognized to be a single proton. Subatomic particles are particles that are smaller than atoms. A positively charged subatomic particle 3. Neutrons have no electrical charge. A subatomic particle with no charge s.. Protons have a positive (+) charge.

There was a time when it was believed that atoms were the fundamental particles of matter and that they could not be further … Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. All matter in this universe is composed of atoms... Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

Neutrons have no electrical charge... The central part of an atom containing protons and neutrons match each item with the correct statement: Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. Total of protons and neutrons in the.

Subatomic particles are particles that are smaller than atoms. . All matter in this universe is composed of atoms.

Total of protons and neutrons in the.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Atoms and molecules are called microscopic particles. A positively charged subatomic particle 3. A subatomic particle with no charge s.

A negatively charged subatomic particle g. Typically in an atom, which two subatomic particles are equal in number? Neutrons have no electrical charge.. The central part of an atom containing protons and neutrons match each item with the correct statement:
:max_bytes(150000):strip_icc()/atom-157646042-584ee6bb5f9b58a8cd2fcb02.jpg)